
10 min read
What is a DiGA and why is it worth creating?
In recent years, we have observed the development of digital therapeutics (DTx) – software supporting patients through health issues. However, the growing number of health apps requires regulation. One of the first countries in Europe to take such action is Germany. Hence, today, we will talk about DiGA.
What will you find in this article?
- Definition of DiGA and DiGA Directory
- List of requirements for DiGACase of DiGA and American Cloud Providers
- DiGA and DiPA differences
- Challenges of creating a DiGA
- A proposition for reading material
What is a DiGA?
As we read on the BfArM (German Federal Institute for Drugs and Medical Devices) website, DiGA (Digitale Gesundheitsanwendungen) is a digital health app on prescription, considered a medical device. The aim of DiGA is to “support the recognition, monitoring, treatment, or alleviation of diseases or support the recognition, monitoring, treatment, or alleviation compensation of injuries or disabilities”.
Some examples? Think about the app that might help patients control their IBS (Irritable Bowel Syndrome) diet or an app that supports overcoming burnout through gamification and guided meditation recordings. To name some real-life solutions – NeuroNation (brain training), My7steps GmbH (mental health support), and Oviva AG (dietary add).
What is the DiGA Directory?
The DiGA Directory is a register of all DiGAs approved by BfArM. Once your app is listed there (permanently or preliminarily), doctors can prescribe it to the patients.
Every DiGA in the Directory is described in terms of its aim, cost, date of adding to the register, type of device you need to use it, and more. The Directory provides a high level of transparency; thus, you can learn much about current DiGAs. We highly encourage you to check the DiGA Directory and find out about interesting digital health apps.
What is DiGAV?
The DiGAV regulation (Digitale Gesundheitsanwendungen-Verordung) came into force on the 21st of April, 2020, and it covers „the requirements and processes for the examination of the eligibility of digital health applications for reimbursement by the state health schemes”. In simple terms, DiGAV is a document stating what requirements a digital health app must meet to be reimbursed and prescribed by doctors to patients in Germany.
The DiGAV itself isn’t a light reading material, but BfArM provides its guide and other materials that make digesting it a little easier. The truth is, if you’re building a DiGA or any Software as a Medical Device, you’ll need to have someone on board who takes care of the regulatory matters or work with external consultants who will guide you through this process.
In any case, Germany may be one of the best places to start if you don’t plan to sell your digital health app directly to the end-users, but rather as a reimbursed medical device (in this case by the German healthcare system or an insurer). How to achieve this?
What are the requirements for a DiGA in Germany?
The DiGA Directory includes only medical apps that meet the highest quality standards. As a result, there are many requirements that a manufacturer must complete for their app to be recognised as DiGA. What should you expect from the DiGA assessment process? Find out below.
What are technical and regulatory requirements for DiGA?
- First of all, DiGA has to meet the criteria stated in the definition – such as:
a. relying on digital technologies,
b. the primary purpose is medical,
c. aiming to support the recognition, monitoring, treatment or alleviation of diseases, injuries or disabilities,
d. not serving primarily a prevention,
e. being shared between a medical provider and a patient (so it focuses on helping patients, not healthcare providers). - You should create a digital health app with a positive care effect for patients. What does it mean? There are two options – your product can provide either:
a. medical benefit (improvement of state of health, the reduction of duration of a disease, the prolongation of survival, or an improvement in the quality of life),
b. patient-relevant improvement of structure and process (seen as a part of the detection, monitoring, treatment or alleviation of disease, aimed at supporting patients’ health behaviour or integrating the processes between patients and healthcare providers, and more – about which you can read here). - Bear in mind that you will have to prove said “positive care effect” with the results of clinical trials. At the latest, you can conduct them within 12 months after provisional listing of your DiGA in the Directory by the BfArM.
- DiGA is supposed to be of the highest medical standards. Hence, you must provide patients with content based on recognised medical knowledge.
- Your app should be interoperable, meaning – as stated by BfArM – “the ability of technical systems to cooperate on a technical-synthetical, semantic, and organisational level”. What does it mean? There should be ease of exchanging data and communication between all sides engaged in using an app in terms of data format and uniform understanding of the information.
- Your app should be easy to use and have an intuitive UI (User Interface), so focus on UX (User Experience) research and tests. Always bear in mind who your target audience is and what digital abilities they might have.
- DiGA should be CE marked as a medical product of risk class I, IIa, or IIb according to MDR or MDD.
- DiGA should be ISO 13485 (rules for manufacturing a high-quality medical device) and ISO 27001 (data safety standards for software developers) certified.
- Patients are supposed to feel safe sharing their private medical data with an app manufacturer. Thus, DiGA data protection has to comply with GDPR (General Data Protection Regulation) – meaning users must consent to processing personal data, have the right to be forgotten, and more.
- You will have to prove the safety of the medical device as stated in recommendations of the Federal Office for Information Security (standards BSI 200-1, BSI 200-2, and BSI 200-3).
Seems like a lot? Don’t be concerned! The DiGAV contains a checklist you can use to verify whether your software complies with all the IT security, quality, and interoperability requirements.
If you check all the boxes and your SaMD has positively gone through MDR certification (class I, IIa, IIb – so, low and medium-risk to the patient), you are eligible to go through a „fast track” assessment procedure and become listed in the DiGA Directory in just 3 months. Easy, right?
DiGA and American Cloud Providers
As you have probably noticed, the safety of patients' data is crucial when it comes to DiGA. Thus, you should be aware of the issue concerning American Cloud Providers (like Amazon Web Services, Microsoft Azure or Google Cloud).
In July 2020, the Court of Justice of the European Union (CJEU) declared that the EU-US Privacy Shield – which regulated exchanges of personal data between the EU and the US – is invalid. What does it mean? In a nutshell, it was not permitted to process medical data in the USA; thus, manufacturers of DiGAs need to maintain patients' data differently.
However, on 10th of July 2023, the European Commission adopted an adequacy decision for the EU-US Data Privacy Framework (DPF). Therefore, it is safe to store data in the USA as long as American Cloud Providers deliver adequate data protection certified through the DPF website. Which companies are certified? To name some of them – Amazon Web Services, Microsoft Azure, and Google Cloud Platform. You can find all participants of Data Privacy Framework here.
To sum up, the cloud platform of your choice should comply with GDPR, meaning there is a need to undertake specific measures to protect said data. What kind of measures? You will find more information in our article on digital health data in GDPR. In addition; you can learn about data processing outside of Germany on the BfArM website.
Warning:
Remember to check how you will store the medical data of the patients. Cloud platforms should comply with GDPR!
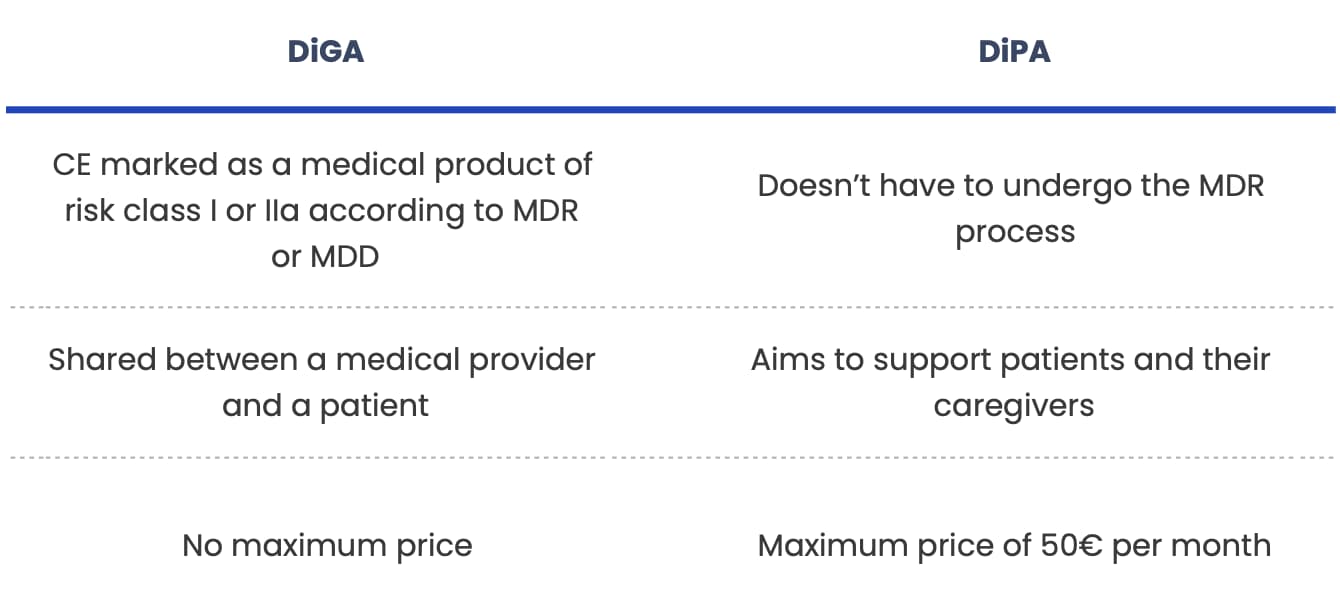
DiGA and DiPA – what’s the difference?
You already know what DiGA is, but have you ever heard about DiPA? DiPA (Digitale Pflegeanwendungen) means digital nursing applications.
DiPAs are supposed to serve people in terms of monitoring and improving their health (hence, “nursing applications”). Those apps can be considered – as BfArM states – “digital assistants”, which can maintain everyday care of a patient by either a family member or a nursing professional.
DiPAs, same as DiGAs, can be reimbursed in Germany. But what’s the difference between them?
DiPA:
- doesn’t have to be a medical device by law and undergo the MDR process (although it can),
- mainly aims to support patients and their caregivers,
- has a maximum price – it can only be reimbursed up to 50 euros per month.
Warning:
You can find a DiPA guide here (unfortunately, at the moment it’s only available in German).
Challenges of creating DiGA
Although creating DiGA is fascinating and rewarding, you may face a few challenges. What are they? How to overcome them? Let us take you through the process of assessing the opportunities and risks of working on a digital app on prescription.
- Getting through the DiGA process
You may feel anxious when you read about the procedure of getting DiGA. It may seem that you have to undertake many activities and don't know how to figure them out. Don't worry! As you can read in the interview with Michał Ryś from ABAStroke, having your medical app in the DiGA Directory is achievable – you just need to follow the instructions provided by BfArM.
Moreover, you can always rely on the help of specialists in medical app certification like us. - Reaching out to doctors
Once your medical app is registered in the DiGA Directory, you need to put much effort into getting doctors to know about your product. How to do it? You should take some time to research what methods of contact work best for your medical field.
What can it be? One way of selling a DiGA is an 'old-pharma style', i.e. classic door-to-door sales through representatives. Many specialists claim that it is the best way of getting doctors to know about your DiGA in Germany; however, it is a time-consuming and costly process. You can also consider creating a webinar for doctors or attending medical conferences in order to teach doctors about your product. - Encouraging doctors to prescribe DiGA
Despite your hard work on DiGA, some doctors might refrain from prescribing apps. They can be biased against modern healthcare technologies and not believe the app will fulfil its promises.
In such a situation, you can undertake activities presenting the benefits of your app to the patients. Consider using testimonials from other doctors and users of your DiGA. Moreover, always remember to show doctors transparent results of clinical trials based on EMB (evidence-based medicine). Over time, this might lead to more doctors prescribing your product. - Educating patients
Another problem that using medical apps may only convince some patients. Others may fear that they won't be able to use it or doubt its effectiveness. However, sometimes the problem might be more straightforward – patients just need to learn about the existence of your DiGA.
Therefore, we suggest conducting educational actions aimed not only at healthcare providers but also at patients. Let them learn more about your product. This way, they will have more confidence to ask the doctor to prescribe your DiGA during their visit.
Reimbursement costs of DiGA
The biggest challenge you will face when creating DiGA deserves to be described separately. One of the tasks you will have to undertake is negotiating the price of DiGA with the German National Association of Statutory Health Insurance Funds (GKV).
What happens during those negotiations? You – an app manufacturer – and GKV discuss the price of DiGA that the health insurance will reimburse. There are usually three or four meetings but if you don’t get into the agreement during that time, an arbitrator will set the price.
Mostly, after finishing the negotiations, the price proposed by the manufacturer is cut. In some cases, prices set by the manufacturer and decided by the board can differ roughly up to 70%, as some claim that the DiGAs’ creators overestimate their costs.
We recommend getting ready for those negotiations – this will help you confidently explain why you've chosen your specific price. Check out the prices of other DIGAs (such as prices in other countries, industry-specific reports, and more) and take your time putting together a line of argument – it will pay off.
Where to start with DiGA?
Building a certifiable digital health application is not a piece of cake. Nor should it be, as – in the least – it’s supposed to help people live better, healthier lives. Consequently, it must meet certain standards and if you're a current or future DiGA creator, it might be helpful to be familiar with these standards and understand what people hope to see from you, your team, and your technology.
There are quite a few places where you can read up on what DiGAs are and how to best prepare to launch one. But there are three we think you should begin with. They will guide you through the entire DiGA creation and development process, and point you in the right direction when it comes to things like regulatory matters, technical requirements, the design of clinical studies, and much more.
- The Fast-Track Process for Digital Health Applications (DiGA) according to Section 139e SGB V – A Guide for Manufacturers, Service Providers and Users – official DiGA guide from the German Federal Institute for Drugs and Medical Devices (BfArM).
- DiGA VADEMECUM – How to Launch Digital Health Apps in the German Healthcare System – an encyclopedia of knowledge on everything from how the German healthcare ecosystem has evolved over the years, to legal requirements of becoming a DiGA, suggestions on how to approach software development, and tips on getting through to the healthcare professionals, institutions, and patients.
- BfArM’s website with up-to-date information about DiGA (if you’re looking for the freshest ones, they will most likely be in German – but Google Translate deals with German-to-English translation very well), DiGA directory (where you can check out what kinds of apps are already listed and refunded as DiGAs), and some great resources for anyone thinking of launching a digital health application in Germany.
If all of this seems a little overwhelming, or it’s not but you would still like to talk to somebody about the regulatory and technical aspects of developing a digital health application – we’re here for you. With many great specialists on board (including our Regulatory Affairs Team), ISO13485-certified processes, and experience with clients like ABAStroke, we’re happy to share our know-how with you.
Or if you prefer, we can just have a talk – we’re always curious about new digital health projects, and maybe we can share some latest tips with you!



